Probing the prion hydration by Molecular dynamics simulations: from native via misfolded to amyloid conformations
Presenter
December 8, 2008
Keywords:
- Molecular dynamics
MSC:
- 92E10
Abstract
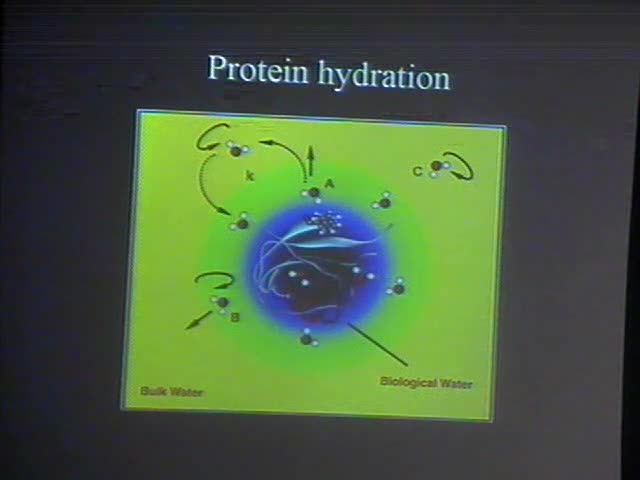
Water at the surface of proteins plays a crucial biological
role. The study of hydration is therefore fundamental for
achieving a complete description of key factors determining the
protein biology. In this framework, Molecular Dynamics
simulations have provided precious tools for elucidating the
structure and dynamics of waters in the protein hydration
shell. We employed Molecular Dynamics to address on the
hydration properties of the Prion Protein, whose misfolding and
aggregation is associated to Transmissible Spongiform
Encephalopathies. The investigations focused on different
states along a likely aggregation pathway specifically
analyzing native state, misfolded state and amyloid-like state.
The presentation will discuss on the influence that water
exerts on protein structural stability [1, 2], intermolecular
interactions [3], misfolding [4] and self-assembly [5, 6].
References
1. PNAS (2005) 102:7535-7540.
2. FEBS Letters (2006) 580:2488-2494.
3. Biophysical Journal (2006) 90:3052-61.
4. Biophysical Journal (2007) 93:1284-1292.
5. Proteins (2008) 70:863-872.
6. BBRC (2008) 366:800-806.